Note4Students
From UPSC perspective, the following things are important :
Prelims level: Uranium isotopes, Radioactivity
Mains level: NA
Physicists in Japan have discovered a new isotope of uranium, with atomic number 92 and mass number 241.
Uranium
- Uranium is a naturally occurring chemical element with the symbol U and atomic number 92.
- It is a heavy metal that is radioactive and found in small quantities in rocks and soils worldwide.
- Uranium has several isotopes, which are atoms that have the same number of protons but different numbers of neutrons.
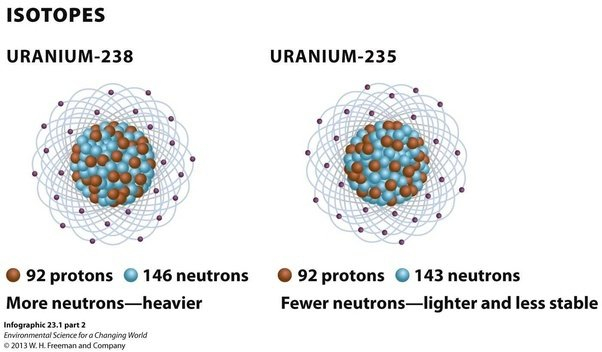
Isotopes of Uranium
The most common isotopes of uranium are uranium-238 and uranium-235.
- Uranium-238: It is the most abundant isotope of uranium, accounting for over 99% of natural uranium. It has 92 protons and 146 neutrons in its nucleus. It is not fissile, which means it cannot sustain a nuclear chain reaction. However, it is fertile, which means it can absorb neutrons and undergo radioactive decay to produce other isotopes such as plutonium-239, which is fissile.
- Uranium-235: It is the second most abundant isotope of uranium, accounting for less than 1% of natural uranium. It has 92 protons and 143 neutrons in its nucleus. Unlike uranium-238, it is fissile, which means it can sustain a nuclear chain reaction. It is used as fuel in nuclear reactors and as the primary material for nuclear weapons.
How are isotopes created?
- Isotopes can be created through natural processes or artificial processes in a laboratory.
- Isotopes are created through natural processes such as radioactive decay, cosmic ray interactions, and nuclear fusion reactions in stars.
- For example, carbon-14 is created in the Earth’s upper atmosphere when cosmic rays interact with nitrogen atoms.
- Isotopes can also be created artificially through nuclear reactions.
- This involves bombarding atoms with particles such as protons, neutrons, or alpha particles, which can change the number of protons and/or neutrons in the nucleus.
How uranium-241 was found?
- To find uranium-241, the researchers accelerated uranium-238 nuclei into plutonium-198 nuclei using the KEK Isotope Separation System (KISS).
- In a process called multinucleon transfer, the two isotopes exchanged protons and neutrons, resulting in nuclear fragments with different isotopes.
- The researchers identified uranium-241 and measured the mass of its nucleus using time-of-flight mass spectrometry.
- Theoretical calculations suggest that uranium-241 could have a half-life of 40 minutes.
Significance of the discovery
- The discovery is significant because it refines our understanding of nuclear physics, particularly the shapes of large nuclei of heavy elements and how often they occur.
- This information helps physicists to design models for nuclear power plants and exploding stars.
Also, what are Magic numbers?
- There is a particular interest in ‘magic number’ nuclei, which contain a certain number of protons or neutrons that result in a highly stable nucleus.
- Lead (82 protons) is the heaviest known ‘magic’ nucleus, and physicists have been trying to find the next element with magic numbers.
- The researchers hope to extend their systematic mass measurements towards many neutron-rich isotopes, at least to neutron number 152, where a new ‘magic number’ is expected.
Conclusion
- The discovery of the new neutron-rich uranium isotope is a major breakthrough in nuclear physics, as it provides essential information for understanding the behavior of heavy elements.
- The researchers’ aim to extend their measurements to other neutron-rich isotopes reflects their commitment to exploring the frontiers of nuclear science and to improve our understanding of the universe.
- Discovering new magic number nuclei through these measurements could have practical applications in designing safer and more efficient nuclear power plants and understanding the properties of exploding stars.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024

