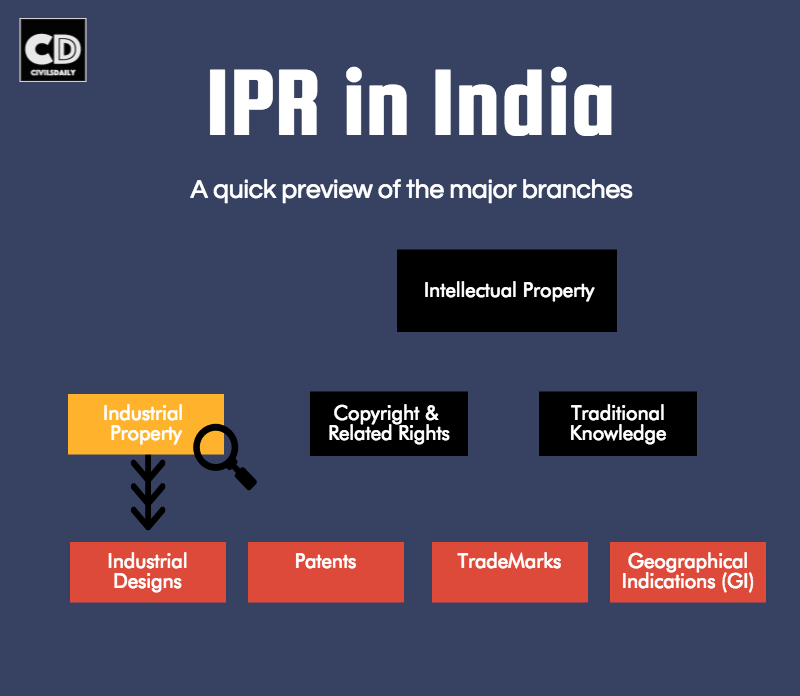
Intellectual Property Rights in India
The Copyright Act, 1957
From UPSC perspective, the following things are important :
Prelims level: Copyright Act
Why in the News?
The ongoing copyright dispute between actors Dhanush and Nayanthara highlights the complexities of copyright law and its potential misuse in the film industry.
What is the dispute?
|
About The Copyright Act, 1957:
| Details | |
| What is it? | • Enacted in 1957 to protect creators’ rights over original works. • Objective: To encourage creativity while balancing public access. • Grants exclusive rights to authors and creators for reproduction, distribution, and public performance of their works. • The Act has been amended to address digital content and technological advancements. |
| Features and Provisions of the Act | • Scope: Covers literary, musical, artistic works, cinematograph films, sound recordings, and more. • Duration: Copyright lasts life of author + 60 years for literary, musical, and dramatic works, and 60 years for films/sound recordings. • Exclusive Rights: Right to reproduce, distribute, perform, and adapt works. • Moral Rights: Includes right to attribution and integrity of the work. |
| What does Section 52 say? | • Section 52 lists exceptions allowing use of copyrighted works without permission in specific situations. • Fair Use: For criticism, review, news reporting, teaching, research, and private use. • Includes exceptions for libraries, archives, and government use. • Important for education and public access—enables non-commercial use of works. |
PYQ:[2014] In a globalized world, Intellectual Property Rights assume significance and are a source of litigation. Broadly distinguish between the terms—Copyrights, Patents and Trade Secrets. [2017] With reference to the ‘National Intellectual Property Rights Policy’, consider the following statements:
Which of the above statements is/are correct? (a) 1 only |
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
What was the tussle over Covaxin IPR?
From UPSC perspective, the following things are important :
Prelims level: Patent process in India, COVAXIN
Why in the News?
- Bharat Biotech International Limited (BBIL), maker of the indigenous coronavirus vaccine Covaxin, admitted to an “inadvertent error” in patent filings. The error involved failing to include scientists from the Indian Council of Medical Research (ICMR) as co-inventors in the patent filings.
COVAXIN Story
|
Vaccine Patents in India
- In India, patents, including those for vaccines, are governed by the Patents Act, 1970, and its subsequent amendments.
- This act aligns with the TRIPS Agreement (Trade-Related Aspects of Intellectual Property Rights) under the World Trade Organization (WTO).
Key Provisions of the Patents Act, 1970:
- Patentable Inventions:
-
-
- An invention must be novel, involve an inventive step, and be capable of industrial application.
- Section 3 of the Patents Act outlines what are not considered inventions, which includes methods of treatment, and processes for medicinal, surgical, curative, prophylactic, diagnostic, therapeutic, or other treatments of human beings.
-
- India grants both process and product patents:
- Product Patents: Grant a monopoly over a specific drug.
- Process Patents: Prevent competitors from using the same sequence of steps to create a similar product.
- Compulsory Licensing:
-
- Under Section 84, compulsory licenses can be issued if the patented invention is not available to the public at a reasonably affordable price, or if the reasonable requirements of the public are not being met.
- Bolar Provision:
-
- Section 107A allows the use of patented inventions, including vaccines, for the purpose of research and development to obtain regulatory approval before the patent expires.
Why was the ICMR not included?
- Bharat Biotech initially excluded ICMR from patent applications because they viewed the ICMR’s role primarily as providing virus strains and conducting clinical trials, rather than being directly involved in the technical processes of vaccine development.
- There might have been a miscommunication or oversight regarding the understanding of intellectual property rights and inventorship between BBIL and ICMR initially.
PYQ:[2013] Bringing out the circumstances in 2005 which forced amendment to the section 3(d) in Indian Patent Law, 1970, discuss how it has been utilized by the Supreme Court in its judgement in rejecting Novartis’ patent application for ‘Glivec’. Discuss briefly the pros and cons of the decision. (200 words) [2014] In a globalized world, Intellectual Property Rights assume significance and are a source of litigation. Broadly distinguish between the terms—Copyrights, Patents and Trade Secrets. |
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Patent (Amendment) Rules, 2024: Key Highlights
From UPSC perspective, the following things are important :
Prelims level: Trademarks, Patents
Mains level: Patent Amendments Rules
In the news-
- The Patent (Amendment) Rules, 2024 were recently published in the Gazette of India, making crucial changes in the Indian patent regime.
Context:
2023 emerged as a landmark year for intellectual property rights (IPR) in India, reflecting the nation’s commitment to innovation and creativity.
|
Key Amendments Introduced:
- Revised Timeline for Request for Examination: The period for submitting a Request for Examination (RFE) in a patent application has been shortened from 48 months to 31 months from the earliest priority date.
- Streamlined Applications: Patent applicants now need to furnish details of corresponding applications solely twice using Form 3.
- Introduction of ‘Certificate of Inventorship’: This new provision acknowledges the contributions of inventors to patented innovations.
- Reduction in Advance Renewal Fees: A discount of 10% on renewal fees is offered if paid electronically in advance for a minimum of four years.
- Decreased Frequency of Patent Working Statements: The requirement to file statements of working patents has been reduced from annually to once every three financial years.
- Enhanced Authority of Controller: The Controller is now empowered to extend specified periods and excuse delays for up to six months.
- Amendments to Opposition Procedures: Adjustments have been made to the time frames for submitting recommendations by an Opposition Board and the response period for applicants in both pre-grant and post-grant opposition procedures.
What are Patents?
- A patent is a legal right granted by a government to an inventor or assignee, giving them exclusive rights to an invention for a limited period.
- It provides the inventor with the right to exclude others from making, using, selling, or importing the patented invention without their permission.
- In essence, a patent acts as a form of intellectual property protection for inventions, allowing inventors to control and commercialize their creations.
- Patents are territorial rights. In general, the exclusive rights are only applicable in the country or region in which a patent has been filed and granted.
Indian Patent Regime: A Backgrounder
- Indian patents are governed by the Indian Patent Act of 1970.
- India has gradually aligned itself with international regimes pertaining to intellectual property rights.
- In 1995, India became a party to the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement following its membership to the World Trade Organisation on January 1, 1995.
- An interesting point is that the original Indian Patents Act did NOT grant patent protection to pharmaceutical products to ensure that medicines were available at a low price.
- Patent protection of pharmaceuticals were re-introduced after the 2005 amendment to comply with TRIPS.
Filing a Patent: Key Terms
- Patentable Subject Matter: Under the Indian Patents Act, inventions related to products, processes, methods, and applications in all fields of technology are patentable, provided they are novel, involve an inventive step, and are capable of industrial application.
- Patent Office: The Indian Patent Office, under the Department for Promotion of Industry and Internal Trade (DPIIT), administers the patent system in India. It operates through four branches located in Kolkata, Mumbai, Delhi, and Chennai, with the Controller General of Patents, Designs & Trade Marks overseeing patent-related matters.
- 20-Year Validity: Patent protection is granted for a limited period, generally 20 years from the filing date of the application.
Various Agreements
India is also a signatory to several IPR-related conventions, including-
- Berne Convention (1886) The Berne Convention for the Protection of Literary and Artistic Works, established in 1886, is an international treaty governing copyright.
- Budapest Treaty (1977): It aims to facilitate the international recognition of patents relating to microorganisms by providing a centralized deposit system for the storage and distribution of biological materials.
- Paris Convention for the Protection of Industrial Property (1883): It aims to harmonize and standardize the protection of industrial property, including patents, trademarks, industrial designs, and trade secrets, among its member countries.
- Patent Cooperation Treaty (1970): It is an international treaty administered by the World Intellectual Property Organization (WIPO) to simplify the process of filing patent applications in multiple countries by providing a unified procedure for filing an international patent application.
Back2Basics:
| Patents | Copyright | Trade Secrets | |
| Legal Basis | Patents Act, 1970 | Copyright Act, 1957 | Common law, contracts |
| Duration of Protection | 20 years | Author’s lifetime + 60 years | Indefinite |
| Nature of Protection | Inventions, processes, methods | Literary, artistic, musical works | Confidential information |
| Criteria for Protection | Novelty, Inventiveness | Originality, Fixation | Confidentiality |
| Registration Requirement | Required | Optional (automatic) | None (advisable) |
| Scope of Protection | Technical aspects | Expression of ideas | Unauthorized use or disclosure |
| Enforcement Mechanism | Civil litigation | Civil and criminal actions | Civil litigation |
| International Protection | Patent protection can be sought internationally through the Patent Cooperation Treaty (PCT) and other international agreements | Copyright protection is recognized internationally through the Berne Convention and other treaties | Protection of trade secrets can vary internationally and may depend on the laws and regulations of individual countries |
| Examples | Inventions, software | Books, music, software | Formulas, processes |
PYQ:
2013: Bringing out the circumstances in 2005 which forced an amendment to section 3(d) in Indian Patent Law, 1970, discuss how it has been utilized by the Supreme Court in its judgement in rejecting Novartis’ patent application for ‘Glivec’. Discuss briefly the pros and cons of the decision. (200 words)
2014: In a globalized world, Intellectual Property Rights assume significance and are a source of litigation. Broadly distinguish between the terms—Copyrights, Patents and Trade Secrets.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Passing Off under Trademark Rules
From UPSC perspective, the following things are important :
Prelims level: Trademarks, Patents
Mains level: Not Much
Why in the news?
- A lady in New Delhi successfully obtained trademark for her Momos brand from New Delhi High Court, after a similar trademark infringed upon her rights and reputation.
- The lady’s legal action invoked ‘passing off’ provisions, seeking cancellation of the infringers’ trademark under relevant sections of the Trademarks Act.
What are Trademarks?
- A trademark is a symbol, design, word, or phrase that is identified with a business. Registering a trademark allows its owner to claim “exclusive rights” to its usage.
- The Trademarks Act of 1999 governs the regime of trademarks and their registration in India.
- It guarantees protection for trademarks registered with the Controller General of Patents, Designs, and Trademarks, also known as the trademark registry.
- According to Section 25 of the 1999 Act, once registered, a trademark is valid for 10 years and can be renewed by the owner periodically.
Concept of ‘Passing Off’
- ‘Passing off’ entails deceptive practices where one brand attempts to profit from the reputation of another through misrepresentation.
- In Cadila Healthcare Limited vs. Cadila Pharmaceuticals Limited (2001), the Supreme Court defined passing-off as a form of unfair trade competition, where one brand seeks to profit from the established reputation of another through deceptive means.
- Infringed parties can seek injunctions, damages, or accounts against the infringing entity to mitigate the damages caused.
Application in the Present Case: Grounds for Trademark Refusal
- Legal Provisions: Sections 11(1), 11(2), 11(3)(a), and 47 of the Trademarks Act outline grounds for refusal to register trademarks and provisions for removal from the register.
- Likelihood of Confusion: Trademarks resembling earlier trademarks, leading to public confusion, are ineligible for registration under Section 11(1).
- Protection of Distinctive Marks: Section 11(2) prohibits registration of marks that take unfair advantage of or harm the reputation of well-known trademarks.
- Non-Compliance and Non-Usage: Section 47 allows removal of trademarks from the register for non-compliance or non-use, subject to aggrieved parties’ applications.
Back2Basics: Trademarks vs. Patents
| Trademark | Patent | |
| Purpose | Identify and distinguish goods or services | Protect new and inventive products or processes |
| Laws and Provisions | Trademarks Act, 1999 | Patents Act, 1970 |
| Subject Matter | Signs like logos, brand names, slogans, packaging | Inventions including products, processes, methods |
| Duration of Protection | 10 years.
Indefinite with periodic renewal |
Typically 20 years from the filing date |
| Registration Process | File application with Trademarks Registry (i.e. Controller General of Patents) | File application with Indian Patent Office |
| Rights Granted | Exclusive use of the trademark in connection with goods or services | Exclusive rights to exploit the invention commercially |
PYQ:
Consider the following statements:
- According to the Indian Patents Act, a biological process to create a seed can be patented in India.
- In India, there is no Intellectual Property Appellate Board.
- Plant varieties are not eligible to be patented in India.
Which of the statements given above is/are correct?
- 1 and 3 only
- 2 and 3 only
- 3 only
- 1, 2 and 3
Practice MCQ:
With reference to Trademarks in India, consider the following statements:
- Trademark can be a symbol, design, word or even a phrase.
- It allows its owner to claim “exclusive rights” to its usage
- It is valid for 5 years.
How many of the given statements is/are correct?
- One
- Two
- Three
- None
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Copyright Protection for Religious Texts
From UPSC perspective, the following things are important :
Prelims level: Copyright Act of 1957
Mains level: Not Much

Central Idea
- The recent ruling by the Delhi High Court has brought attention to copyright infringement concerning religious texts, particularly the Bhaktivedanta Book Trust’s works on Indian religious philosophy and spiritualism.
- This landmark case addresses copyright protection for sacred texts and the implications for digital platforms.
- Let’s explore the details of the case and its broader implications.
Are Religious Texts Copyright-Protected?
- Public Domain: Most religious scriptures, such as the Old Testament and New Testament, are in the public domain. Copyright law does not apply to works in the public domain.
- Exceptions: Modern translations of religious texts, like the New International Version (NIV) of the Bible, may enjoy copyright protection as they represent new creative works by translators.
- Protections: Additionally, transformative works, like television adaptations of epics like the Ramayana and Mahabharata, are protected.
Understanding Copyright Law in India
- Scope of Protection: The Indian Copyright Act of 1957 safeguards “original work,” creative expressions independently created and fixed in a tangible medium.
- Exclusive Rights: It grants exclusive rights to creators/authors, including the right to use, reproduce, distribute, perform, and display their work.
- Transformative Works: The Act also protects transformative works, which creatively modify, reinterpret, or build upon existing material to create something distinct.
Duration of Copyright Protection
| Literary, Dramatic, Musical, Artistic Works | Lifetime of the author plus 60 years from the year following the author’s death or last surviving author’s death. |
| Cinematographic Films | 60 years from the year of publication or creation. |
| Sound Recordings | 60 years from the year of first publication. |
| Anonymous or Pseudonymous Works | 60 years from the year of publication, or lifetime of the author plus 60 years if the author’s identity is disclosed during this period. |
Bhaktivedanta Book Trust’s Case
- Founder’s Works: The trust claimed copyright ownership of its founder’s works, which had simplified religious books and scriptures, making them accessible to the common man.
- Infringement Allegation: The trust alleged that various websites, mobile apps, and Instagram handles were reproducing a significant number of its copyrighted works almost verbatim on their online platforms without authorization, constituting infringement.
Delhi High Court’s Ruling
- Copyright Protection: The court ruled that adaptations of sacred scriptures, including explanations, meanings, interpretations, and audio-visual works, are entitled to copyright protection because they represent original works by authors themselves.
- Reproduction Clarification: While the reproduction of the actual text of sacred texts, such as the Srimad Bhagavad Gita, is permissible, the court emphasized that copyright protection applies to the original parts of literary works that preach, teach, or explain the scripture.
- Trust’s Rights: Given that Srila Prabhupada had entrusted the copyrights to be administered by the Bhaktivedanta Book Trust, the court emphasized that the works cannot be reproduced without the trust’s authorization, license, or permission.
- Preventing Piracy: The court acknowledged that unauthorized reproduction, including shlokas (verses), translations, and interpretations, by defendant entities would result in immense revenue loss for the trust.
Conclusion
- The Delhi High Court’s ruling on copyright protection for religious texts has far-reaching implications for safeguarding the originality and rights associated with sacred scriptures.
- While religious texts themselves may not be copyright-protected, creative adaptations, explanations, and interpretations enjoy legal protection.
- This decision serves as a precedent for preserving the intellectual property rights of organizations involved in disseminating spiritual knowledge while discouraging unauthorized reproduction and piracy.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Draft Patent Amendment Rules and Issues
From UPSC perspective, the following things are important :
Prelims level: Draft Patent Amendment Rules
Mains level: Read the attached story

Central Idea
- On August 23, the Department for Promotion of Industry and Internal Trade in India unveiled draft patent amendment rules.
- These changes, if enacted, may have significant implications for pharmaceutical companies and patients, particularly in the global South.
Draft Patent Amendment Rules: Key takeaways
- Financial Burden: A notable modification is the introduction of variable fees for filing pre-grant oppositions, potentially placing a substantial financial burden on civil society organizations and patient groups.
- Maintainability Decision: Of particular concern is the provision granting the controller the authority to determine the maintainability of representation by individuals or civil society organizations seeking to file pre-grant oppositions.
Impact on Public Health Safeguards
- Key Public Health Safeguard: Pre-grant opposition serves as a crucial public health safeguard against practices like patent evergreening and the granting of unwarranted monopolies. It ensures continued accessibility to quality-assured and affordable generic medicines.
- Lobbying for Weakened Safeguards: The draft amendment rules have raised concerns that they may undermine these safeguards and potentially extend patent protection on frivolous grounds. Big pharmaceutical companies have long lobbied to remove critical safeguards from India’s patent laws.
Critiques and Concerns
- Lack of Rational Basis: Critics argue that the rules’ provision for controller-determined maintainability lacks a rational basis and may create more problems. Without clear guidelines, decisions on the eligibility of pre-grant opposition filers could become arbitrary.
- Favouring Corporations: Some believe that the government is aligning with pharmaceutical companies’ interests, as these corporations often seek to limit pre-grant opposition.
- Unique Provision at Risk: Pre-grant opposition, an exceptional provision within the Indian Patent Act, has been crucial in protecting public health interests. Weakening this provision could have dire consequences for patients and the generic drug industry.
Precedents of Successful Opposition
- Past Precedents: Pre-grant opposition filed by patient groups and civil society organizations has led to the rejection of patent extensions pursued by pharmaceutical companies based on weak claims of “novel invention.”
- Notable Instances: Examples include opposition to patents for drugs like Tenofovir disoproxil fumarate (TDF), Nevirapine, Glivec (imatinib mesylate), Zidovudine/Lamivudine (HIV medicines), and Lopinavir/Ritonavir (HIV medicines).
Potential Ramifications
- Global Implications: The proposed changes could disproportionately impact patients in India and the global South, who heavily rely on India’s production of affordable generic drugs and vaccines.
- Threat to Access: Weakening pre-grant opposition may impede access to essential medicines, putting patients at risk and affecting the generic drug industry.
- Concerns Raised: Experts emphasize that any erosion of this provision within the Indian Patent Act would be a significant change, jeopardizing patients’ ability to access affordable medications and enabling pharmaceutical corporations to exert greater control over the market.
Conclusion
- The draft patent amendment rules have sparked concerns that they may undermine essential safeguards, potentially benefiting pharmaceutical giants while posing a threat to patients’ access to affordable medicines.
- The pivotal role of pre-grant opposition in safeguarding public health interests is at risk, raising questions about the impact on patients in India and beyond.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
What is the Open-Source Seeds Movement?
From UPSC perspective, the following things are important :
Prelims level: Plant Breeders' Rights, Open Source Seeds
Mains level: Not Much

Central idea
- The article discusses the concept of open-source software and its parallels with open-source seeds.
- Richard Stallman pioneered the Free Software Movement and developed the General Public License (GPL) to protect users’ rights and prevent code misappropriation.
- Farmers have been innovating and sharing seeds without intellectual property rights (IPR) claims for centuries.
Backgrounder: Plant Breeders’ Rights
- Plant breeders’ rights (PBR) granted exclusive rights to breeders and developers of new varieties.
- Farmers’ rights were limited under this regime.
- The TRIPS agreement established a global IPR regime over plant varieties.
- The consolidation of the seed sector raised concerns about the freedom to innovate.
Forms of IPR Protection in Agriculture
- There are now two forms of IPR protection in agriculture: PBR and patents.
- Together, they restrict farmers’ rights and the freedom to develop new varieties.
- The use of genetically modified seeds and IP claims triggered many problems, including State intervention on Bt cotton seeds in India.
- The decline of public sector breeding and the dominance of the private sector in the seed sector increased the need for alternatives.
What are Open Source Seeds?
- The success of open-source software inspired a solution for seeds.
- In 1999, a Canadian plant breeder named T.E. Michaels suggested an approach to seeds based on the principles of open-source software.
- In 2012, Jack Kloppenburg launched the Open Source Seeds Initiative (OSSI) in Wisconsin.
- Agrecol launched another initiative in Europe, and similar programs have come up worldwide.
Open Source Seeds Initiatives in India
- In India, the Hyderabad-based Centre for Sustainable Agriculture (CSA), part of the Apna Beej Network, developed a model incorporated into an agreement between CSA and the recipient of the seed/germplasm.
- CSA’s Open Source Seeds Initiative uses a contracts approach similar to Agrecol’s strategy.
- The number of seed firms using open-source models and the crop varieties and seeds made available thereunder is small but growing.
- India is yet to test and adopt it widely.
Potential Applications of Open-Source Seeds
- Open-source principles can help promote farmer-led participatory plant-breeding exercises.
- Traditional varieties often lack uniformity and aren’t of excellent quality, but open-source principles can facilitate testing, improvisation, and adoption.
- Open-source principles can be used in farmer-led seed conservation and distribution systems.
- The government and other stakeholders can consider adopting this approach to more widely adopt traditional varieties.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
What are Performer’s Rights?
From UPSC perspective, the following things are important :
Prelims level: Performers' Right
Mains level: Not Much
Central idea
- This article discusses a recent case involving Bollywood actor and producer, in which the Bombay High Court ruled that sales tax cannot be levied on the transfer of copyright.
- The ruling has implications for the entertainment industry, particularly in terms of taxation and copyright protection as well as performer’s rights.
What are Performer’s Rights?
- It refer to the legal rights granted to performing artists or performers in relation to their performances.
- These rights generally include the right to control and protect their performances from unauthorized use, reproduction, distribution, and public performance.
- Performer’s rights may include the right to control the following:
- Recording: Performers have the right to prevent others from recording their live performances without their consent.
- Broadcasting and Communication to the Public: Performers have the right to control the broadcasting, communication, and distribution of their performances to the public, including radio, television, and online streaming platforms.
- Reproduction: Performers have the right to control the reproduction of their performances in any media format.
- Adaptation: Performers have the right to control the adaptation of their performances into other forms, such as musicals or films.
- Attribution: Performers have the right to be identified as the performers of their works, and to prevent others from falsely claiming authorship of their performances.
Legal protection of performer’s right
Legal protection of performers’ rights has evolved over time through international treaties and national laws.
- The Rome Convention in 1961 was the first significant development in the protection of performers’ rights.
- Performers’ rights are protected under various international treaties such as the Rome Convention and the WIPO Performances and Phonograms Treaty.
- In 1996, WIPO Performance and Phonogram Treaty (WPPT) recognized the moral rights of performers for the first time in any international treaty.
- In India, performer rights were recognized under the Copyright Act of 1957 in 1994.
- The Copyright Act is in conformity with the WIPO Copyright Treaty (WCT) and the WIPO Performances and Phonograms Treaty (WPPT), both concluded in 1996.
- The protection of performers’ rights in India lasts for 50 years from the end of the year in which the performance was fixed or took place.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
What is Patent Evergreening?
From UPSC perspective, the following things are important :
Prelims level: Patent Evergreening
Mains level: Read the attached story

Central idea: Patent Evergreening
- Indian Patent Office rejects Johnson & Johnson’s attempt to extend monopoly on manufacturing Bedaquiline in India beyond July 2023.
- This is a victory for patients fighting for wider access to crucial anti-tuberculosis drug Bedaquiline.
- Expired primary patents pave the way for generic drug manufacturers to produce Bedaquiline, thus ensuring cheaper and wider access to the drug.
Significance of the move
- The drug has been shown to have a high success rate in treating MDR-TB, and is considered to be a significant breakthrough in the fight against this disease.
- However, the high cost of the drug has made it difficult for many patients to access it, particularly in developing countries.
What is Bedaquiline?
|
Implications
- India and the US has often been at the crossheads due to Section 3(d) of Patents Act that allows for “generic competition by patenting only novel and genuine inventions.”
- US always accuses India as one of the most challenging major economies as far as IP protection and enforcement is concerned.
Indian Patent Regime: A Backgrounder
- Indian patents are governed by the Indian Patent Act of 1970.
- India has gradually aligned itself with international regimes pertaining to intellectual property rights.
- It became a party to the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement following its membership to the World Trade Organisation on January 1, 1995.
- The interesting point is that the original Indian Patents Act did not grant patent protection to pharmaceutical products to ensure that medicines were available at a low price.
- Patent protection of pharmaceuticals was re-introduced after the 2005 amendment to comply with TRIPS.
What is Patents Evergreening?
- One of the main points of contention between India and the US has been Article 3(d) of the Indian Patent Act.
- Section 3 deals with what does not qualifyas an invention under the Act, and Section 3(d) in particular excludes the mere discovery of a new form of a known substance.
- Section 3(d) prevents the mere discovery of any new property or new use for a known substance from being patented as an invention unless it enhances the efficacy of the substance repetitive.
- This prevents, what is known as “Evergreening” of patents.
- According to the Committee’s report, Section 3(d) allows for “generic competition by patenting only novel and genuine inventions.”
Conclusion
- The gravity of public health problems affecting developing and least developed nations must be recognized by developed nations such as the US.
- Though intellectual property protection is important for the development of new medicines but the right to protect public health and, in particular, to promote access to medicines for all is far more important.
Are you an IAS Worthy Aspirant? Get a reality check with the All India Smash UPSC Scholarship Test
Get upto 100% Scholarship | 900 Registration till now | Only 100 Slots Left
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
India ranks 42 among 55 countries on International IP Index
From UPSC perspective, the following things are important :
Prelims level: International IP Index
Mains level: Patenting in India

India ranks 42nd among 55 leading global economies on the International Intellectual Property (IP) Index released by the US Chambers of Commerce.
International IP Index
- It is released annually by the US Chamber of Commerce.
- The index evaluates IP rights in 55 global economies across 50 unique indicators.
- The indicators include patent and copyright policies to commercialization of IP assets, and ratification of international treaties.
- The index aims to help nations navigate toward a brighter economic future marked by greater innovation, creativity, and competitiveness.
Key prospects for India
- India is ripe to become a leader for emerging markets seeking to transform their economy through IP-driven innovation said the report.
- Successful IP-based businesses in India include pharmaceutical companies, software firms, and creative industries.
Key factors contributing to India’s score
- IP laws
- Efficiency of its judicial system and
- Level of enforcement of IP rights
Challenges faced
- These are some challenges faced by Indian companies in protecting and monetizing their IP include issues such as-
- Counterfeiting
- Piracy
- Weak enforcement of IP laws
IP regime in India
Broadly, the following acts deal with the protection of intellectual property:
- Trade Marks Act, 1999
- The Patents Act, 1970 (as amended in 2005)
- The Copyright Act, 1957
- The Designs Act, 2000
- The Geographical Indications of Goods (Registration and Protection) Act, 1999
- The Semiconductor Integrated Circuits Layout Design Act, 2000
- The Protection of Plant Varieties and Farmers’ Right Act, 2001
- The Information Technology Act, 2000
Way forward
- India must undertake reforms to strengthen IP protection and enforcement, modernizing IP laws, and increasing investment in IP infrastructure.
- Collaboration between government, industry, and academia is important in improving India’s IP ecosystem/
- Lessons can be learned from other countries with successful IP regimes, such as the United States, Japan, and South Korea.
Attempt UPSC 2024 Smash Scholarship Test | FLAT* 100% OFF on UPSC Foundation & Mentorship programs
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
National IPR Policy: Discussing the rights of all the stakeholders
From UPSC perspective, the following things are important :
Prelims level: IPR policy
Mains level: Intellectual property rights , reforms and concerns
Context
- In May 2016, the then Department of Industrial Policy and Promotion (now known as the Department for Promotion of Industry and Internal Trade) under the Ministry of Commerce released the 32-page National IPR Policy. The overall purpose of this document was to spell out the government’s comprehensive vision for the IPR ecosystem in the country towards shaping a more innovative and creative Bharat.
Crack Prelims 2023! Talk to our Rankers
What is a Patent?
- A patent is an exclusive set of rights granted for an invention, which may be a product or process that provides a new way of doing something or offers a new technical solution to a problem.
Know the basics: Intellectual Property rights (IPR)
- IPR refers to the legal rights that protect an individual’s or company’s creations and inventions (such as inventions, literature, music, and symbols) from being used or copied by others without permission.
- IP is protected in law by, for example, patents, copyright and trademarks, which enable people to earn recognition or financial benefit from what they invent or create.
- By striking the right balance between the interests of innovators and the wider public interest, the IP system aims to foster an environment in which creativity and innovation can flourish.
Three important objectives of National IPR policy document
- Strong and effective IPR laws: Under the head Legal and Legislative Framework, the goal was to have strong and effective IPR laws, which balance the interests of right owners with larger public interest.
- Modernise and strengthen IPR administration: Under Administration and Management, the objective was to modernise and strengthen service-oriented IPR administration; and
- Strengthening adjudicatory mechanism: Under Enforcement and Adjudication, the focus was to strengthen the enforcement and adjudicatory mechanisms for combating IPR infringements.
Changes in IPR ecosystem so far
- Structural and legislative changes: Over the last six years, the IPR ecosystem in this country has witnessed both structural and legislative changes.
- Intellectual Property Appellate Board (IPAB): IPAB was dissolved in April 2021 as part of tribunal reforms, and its jurisdiction was re-transferred to high courts.
- Dedicated IP Division: This was followed by the establishment of dedicated IP benches the IP Division by the Delhi High Court, arguably the country’s leading court on the IPR front, for speedier disposal of IPR disputes.
- IP friendly environment: Such measures, one presumes, are intended to convey to investors and innovators that Bharat is an IP-savvy and even IP-friendly jurisdiction without compromising on national interest and public health commitments.
- For instance: This is evident from the very same National IPR Policy which, among other things, expressly recognises the contribution of the Indian pharmaceutical sector in enabling access to affordable medicines globally and its transformation to being the pharmacy of the world.
What are the concerns?
- Patent-friendliness, rather patentee-friendliness: It appears that the patent establishment of the country has drawn a very different message it has gone on an overdrive to prove its patent-friendliness, rather patentee-friendliness, in the pharmaceutical sector at the expense of public health and national interest respectively.
- Evergreening of patents on critical drugs: Evergreening patents on drugs which relate to treatment of diabetes, cancers, cardiovascular diseases and other serious conditions continue to be granted to pharmaceutical innovator companies by the Indian Patent Office.
- Enforcements at the expense of statutory rights: Worse, they are regularly enforced through courts at the expense of the statutory rights of generic manufacturers and to the detriment of patients.
- Unavailability of affordable drugs: The delayed entry of generic versions of off-patent drugs affects adversely the availability of affordable medicines to patients in a lower middle-income country such as Bharat where most middle-class families and below are only a hospital-visit away from dipping into their hard-earned savings.
Way ahead
- It must be understood that IP legislations such as the Patents Act do not exist for the sole benefit of IP right owners.
- Patent bargain is in which the society is expected to benefit from dynamic innovation-based competition between market players.
- Clearly, there are four stakeholders under the Patents Act the society, government, patentees and their competitors.
- Each of these stakeholders has rights under the statute which makes all of them right owners.
- To interpret, apply and enforce the Act to the exclusive benefit of patentees, and that too evergreening patentees, is to abridge and reduce to a naught the legitimate rights of other stakeholders, leading to sub-optimal and worse, anti-competitive market outcomes.
Conclusion
- It is one thing to operate under the understandable belief that Bharat needs to add layers to its IPR ecosystem to attract investment. However, it is entirely another to equate IPR-sensitivity with a pro-patentee position at the expense of public health obligations and long-term national interest. Make in India must be reconciled with Atmanirbhar Bharat, and in the event of conflict between the two, the latter must prevail for Bharat to retain its position as the pharmacy of the world.
Mains question
Q. What is Intellectual property rights? Discuss the changes taken place in India’s IPR ecosystem so far and highlight the concerns.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
What constitutes a Trademark Violation?
From UPSC perspective, the following things are important :
Prelims level: Trademark
Mains level: IP protection in India

The Delhi High Court dismissed a case of trademark infringement brought by the global fast food chain against a Delhi-based restaurant.
What is a trademark?
- A trademark is a symbol, design, word or phrase that is identified with a business.
- When a trademark is registered, its owner can claim “exclusive rights” on its use.
- The Trademark Act, 1999, governs the regime on trademark and its registration.
- The Act guarantees protection for a trademark that is registered with the Controller General of Patents, Designs, and Trademarks, also known as the trademark registry.
- A trademark is valid for 10 years, and can be renewed by the owner indefinitely every 10 years.
Violation of trademark
- Using a registered trademark without authorization of the entity that owns the trademark is a violation or infringement of the trademark.
- Using a substantially similar mark for similar goods or services could also amount to infringement.
- In such cases, courts have to determine whether this can cause confusion for consumers between the two.
- There are several ways in which a trademark can be infringed. However, the trademark owner has to show that the trademark has a distinct character-
- Deceptive similarity: The law states that a mark is considered deceptively similar to another mark if it nearly resembles that other mark, confusing the consumer in the process. Such deception can be caused phonetically, structurally or visually.
- Passing off: Say, a brand logo is misspelt in a way that’s not easy for the consumer to discern. The Supreme Court has ruled that passing off is a “species of unfair trade competition or of actionable unfair trading by which one person, through deception, attempts to obtain an economic benefit of the reputation which other has established for himself in a particular trade or business”.
Crack Prelims 2023! Talk to our Rankers
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Patents in India
From UPSC perspective, the following things are important :
Prelims level: KAPILA scheme
Mains level: IPR regime
 Context
Context
- Increasing the efficiency of processing patent applications and wider academia-industry collaboration are crucial steps for patent system.
What is patent system?
- A patent system is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an enabling disclosure of the invention.
Why are patents important?
- A patent is important because it can help safeguard our invention. It can protect any product, design or process that meets certain specifications according to its originality, practicality, suitability, and utility. In most cases, a patent can protect an invention for up to 20 years.
 How to get patent?
How to get patent?
- To get a patent, technical information about the invention must be disclosed to the public in a patent application.
- The patent owner may give permission to, or license, other parties to use the invention on mutually agreed terms.
- The owner may also sell the right to the invention to someone else, who will then become the new owner of the patent.
- Once a patent expires, the protection ends, and an invention enters the public domain; that is, anyone can commercially exploit the invention without infringing the patent.
Terms of Patent
- Patents may be granted for inventions in any field of technology, from an everyday kitchen utensil to a nanotechnology chip.
- An invention can be a product – such as a chemical compound, or a process, for example – or a process for producing a specific chemical compound.
- Patent protection is granted for a limited period, generally 20 years from the filing date of the application.
- Patents are territorial rights. In general, the exclusive rights are only applicable in the country or region in which a patent has been filed and granted, in accordance with the law of that country or region.
 How patents can support inventors and improve lives
How patents can support inventors and improve lives
- Recognize and reward: Patents recognize and reward inventors for their commercially-successful inventions. As such they serve as an incentive for inventors to invent. With a patent, an inventor or small business knows there is a good chance that they will get a return on the time, effort and money they invested in developing a technology. In sum, it means they can earn a living from their work.
- Economic opportunity: When a new technology comes onto the market, society as a whole stands to benefit – both directly, because it may enable us to do something that was previously not possible, and indirectly in terms of the economic opportunities (business development and employment) that can flow from it.
- Research and development (R&D): The revenues generated from commercially successful patent-protected technologies make it possible to finance further technological research and development (R&D), thereby improving the chances of even better technology becoming available in the future.
- Opportunities for business growth: A patent effectively turns an inventor’s know-how into a commercially tradeable asset, opening up opportunities for business growth and job creation through licensing and joint ventures, for example.
- Commercialization of a technology: Holding a patent also makes a small business more attractive to investors who play a key role in enabling the commercialization of a technology.
- Spark new ideas: The technical information and business intelligence generated by the patenting process can spark new ideas and promote new inventions from which we can all benefit and which may, in turn, qualify for patent protection.
- No freebies: A patent can help stop unscrupulous third parties from free riding on the efforts of the inventor.
What is KAPILA Initiative?
- Full form: KAPILA is an acronym for Kalam Program for IP (Intellectual Property) Literacy and Awareness.
- Guidelines for patent Filing: Under this campaign, students pursuing education in higher educational institutions will get information about the correct system of the application process for patenting their invention and they will be aware of their rights.
- Encouragement to students: The program will facilitate the colleges and institutions to encourage more and more students to file patents.
Thing to remember
Remember one thing, ‘KAPILA’ Program is related to IP awareness. It sounds much like an animal husbandry related initiative.
Way ahead
- As the patent system is a critical aspect of the national innovation ecosystem, investing in the patent ecosystem will help in strengthening the innovation capability of India.
- The right interventions should be made for the promotion of the quality of patent applications and collaboration between academia and industry.
Mains question
Q. A patent can help stop unscrupulous third parties from free riding on the efforts of the inventor. Discuss this statement in context of protection of innovative ecosystem in India.
UPSC 2023 countdown has begun! Get your personal guidance plan now! (Click here)
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Indian Patent Regime vs. US norms
From UPSC perspective, the following things are important :
Prelims level: IPRs, Patents
Mains level: Patent regime of India
The US Trade Representative (USTR) said in a report released last month that India was one of the most challenging major economies as far as IP protection and enforcement is concerned.
What is the news?
- US has decided to retain India on its Priority Watch List along with six other countries —Argentina, Chile, China, Indonesia, Russia and Venezuela.
What is a Patent?
- A patent is an exclusive set of rights granted for an invention, which may be a product or process that provides a new way of doing something or offers a new technical solution to a problem.
Indian Patent Regime: A Backgrounder
- Indian patents are governed by the Indian Patent Act of 1970.
- India has gradually aligned itself with international regimes pertaining to intellectual property rights.
- It became a party to the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement following its membership to the World Trade Organisation on January 1, 1995.
- An interesting point is that the original Indian Patents Act did not grant patent protection to pharmaceutical products to ensure that medicines were available at a low price.
- Patent protection of pharmaceuticals were re-introduced after the 2005 amendment to comply with TRIPS.
Various agreements
- India is also a signatory to several IPR related conventions, including the Berne Convention, which governs copyright.
- It is signatory to the Budapest Treaty, the Paris Convention for the Protection of Industrial Property, and the Patent Cooperation Treaty (PCT), all of which govern various patent-related matters.
Issues raised about India
- Among the issues raised in the report are:
- India’s inconsistencies regarding patent protection
- Concerns about what can be patented
- Waiting time for obtaining patents
- Burdensome reporting requirements and
- Doubts about data safety
- Trademark counterfeiting and secrets
- India had undertaken an intellectual property review exercise last year, where a Parliamentary Standing Committee examined this subject.
Contention of the US: Patents Evergreening
- One of the main points of contention between India and the U.S. has been Article 3(d) of the Indian Patent Act.
- Section 3 deals with what does not qualify as an invention under the Act, and Section 3(d) in particular excludes the mere discovery of a new form of a known substance.
- Section 3(d) prevents the mere discovery of any new property or new use for a known substance from being patented as an invention unless it enhances the efficacy of the substance repetitive.
- This prevents, what is known as “Evergreening” of patents.
- According to the Committee’s report, Section 3(d) allows for “generic competition by patenting only novel and genuine inventions.”
TRIPS and the Doha Declaration
- The Doha Declaration on the TRIPS Agreement and Public Health was adopted on November 14, 2021, by the WTO member states.
- This declaration recognises the gravity of public health problems affecting developing and least developed nations.
- It recognises that “intellectual property protection is important for the development of new medicines,” and acknowledges concerns about its effects on prices.
- It is interpreted and implemented as a right to protect public health and, in particular, to promote access to medicines for all.
Key provisions of Doha Agreement
- Compulsory licences can be invoked by a state in public interest, allowing companies apart from the patent owner to produce a patented product without consent.
- It concluded that India must not compromise on the patentability criteria under Section 3(d).
- It said that this ensures the growth of generic drug makers and the public’s access to affordable medicines.
- It indicated that India should resolve its differences with the US regarding the disqualification of incremental inventions through bilateral dialogue.
Positive steps taken by India
- The USTR report highlighted some positive steps taken by India in the recent past.
- India has accession to the World Intellectual Property Organisation (WIPO) Performances and Phonograms Treaty and WIPO Copyright Treaty, collectively known as the WIPO Internet Treaties, in 2018 and the Nice Agreement in 2019.
Back2Basics: Intellectual Properties
- IP is protected in law by, for example, patents, copyright and trademarks, which enable people to earn recognition or financial benefit from what they invent or create.
- By striking the right balance between the interests of innovators and the wider public interest, the IP system aims to foster an environment in which creativity and innovation can flourish.
Types of IP:
(1) Copyright
- Copyright is a legal term used to describe the rights that creators have over their literary and artistic works.
- Works covered by copyright range from books, music, paintings, sculpture and films, to computer programs, databases, advertisements, maps and technical drawings.
(2) Patents
Discussed above
(3) Trademarks
- A trademark is a sign capable of distinguishing the goods or services of one enterprise from those of other enterprises.
- Trademarks date back to ancient times when artisans used to put their signature or “mark” on their products.
(4) Geographical Indications
- Geographical indications and appellations of origin are signs used on goods that have a specific geographical origin and possess qualities, a reputation or characteristics that are essentially attributable to that place of origin.
- Most commonly, a geographical indication includes the name of the place of origin of the goods.
(5) Trade secrets
- Trade secrets are IP rights on confidential information which may be sold or licensed.
- The unauthorized acquisition, use or disclosure of such secret information in a manner contrary to honest commercial practices by others is regarded as an unfair practice and a violation of the trade secret protection.
UPSC 2023 countdown has begun! Get your personal guidance plan now! (Click here)
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Understanding Software Copyright and Licences
From UPSC perspective, the following things are important :
Prelims level: Software licensing
Mains level: Read the attached story
This newscard is an excerpt from the original article published in The Hindu.
Does software have copyright? Even more specifically, is the Internet free inspite of software copyright? Are software programming languages free of cost? How does copyright apply to software?
Software licensing
- A copyright gives a creator the legal right to own, distribute and profit from his or her creative work.
- There are different kinds of software licences that allow free use of software:
(1) Proprietary License
- There is proprietary software which is to be purchased as a one-time transaction or as yearly licences.
- A popular example is Microsoft Windows which is purchased along with the computer or Microsoft Office which typically has a yearly licence that has to be renewed upon payment.
(2) Creative Commons licence (CC)
- There is the Creative Commons licence (CC) which is public domain: any software or work that is in CC can be used and distributed free of cost.
- For example, Wikipedia is under CC and hence its contents can be used freely with the condition that attribution is made to Wikipedia (this is called ‘Creative Commons – Attribution-ShareAlike).
(3) Permissive Software licence
- Another form of free software licence is Permissive Software licence which is popular in the software developer community and in the commercial world.
- This licence allows free use and modification of software. There are further specific licences under this category, like the Apache licence and MIT licence.
(4) Apache licence
- The Apache licence is maintained by the Apache Software Foundation which is a non-profit entity.
- Many popular and powerful softwares like Spark (used in Big Data) have been developed under Apache licence.
- MIT licence is maintained by the Massachusetts Institute of Technology and it covers hundreds of software packages including GitLab and Dot NET.
What are Open Software?
- All free and permissive software licences are similar to Free and Open Source Software (FOSS).
- This is a set of rules and free software brought under one umbrella in the 1980s by Richard Stallman, a famous computer scientist and activist.
- FOSS maintains its own licence, called GNU GPL (Gnu’s Not Unix General Public Licence) to govern and distribute free software but it comes with restrictions that its adoption and modification be for free use.
- In the software community, ‘open source’ means any of the above non-proprietary licences.
Who maintains open source softwares?
- Open source software packages are developed and maintained by programmers from around the world.
- Until the mid-1990s, the idea of the general public collaborating to create software for free seemed to be unrealistic and confined to small, elite communities.
- However, with the success of a free operating system like Linux (which is under GNU GPL licence), many were convinced that open source could create sophisticated solutions because of access to top programmers around the world.
Is the Internet free?
- To access and to create content on the internet, there are costs involved such as infrastructure costs like network cost and the cost to host and maintain the content.
- However, the core of the internet itself is free: it is free to use ideas like linking contents on the internet, transferring them with a network software protocol and adopting the associated standards like maintaining the website address (Uniform Resource Locator-URL).
Are programming languages free of cost?
- Until the 1980s, popular programming languages had a price but with the advent of Java in the 1990s and thanks to the initiatives of Richard Stallman and his Free Software Foundation in the 1980s, many languages, especially modern ones like Go or popular ones like Python are free.
- Java is somewhere in the middle where there are free implementations of the language that most software developers use but there are also paid implementations provided by Oracle.
- In general, the realisation in the software community is that a free language has widespread adoption and leads to the availability of an expert pool of programmers.
- The last two decades have seen a proliferation of open source software and the future is even more exciting.
UPSC 2022 countdown has begun! Get your personal guidance plan now! (Click here)
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
A blow to equitable access to essential medicines
From UPSC perspective, the following things are important :
Prelims level: Compulsory licencing
Mains level: Paper 3- TRIPS waiver for COVID-19
Context
At the height of the COVID-19 pandemic in October 2020, India and South Africa had tabled a proposal seeking a temporary waiver on COVID-19 related products from the TRIPS. Nearly 18 months later, 164 members of the WTO could not find common ground on the “waiver proposal”.
How will the waiver help?
- The application and enforcement of intellectual property rights (IPRs) are affecting the timely provisioning of affordable medical products to patients.
- Therefore, India and South Africa argued that therefore, argued that “rapid scaling up of manufacturing globally” was “an obvious crucial solution to address the timely availability and affordability of medical products to all countries in need”, and for doing so, IPRs must be waived for at least three years.
The EU solution
- The EU had proposed in a submission in June 2021 that “[c]ompulsory licences are a perfectly legitimate tool that governments may wish to use in the context of a pandemic”.
- India and South Africa, the movers of the “waiver proposal”, are among the four countries that found a “compromise outcome”.
- Only vaccines are included: The solution is a severely truncated version of the “waiver proposal” in terms of product coverage, as only vaccines are included.
- Generally, patent laws, including that of India’s, allow for the grant of compulsory licences if patent holders charge high prices on the proprietary medicines in exercise of their monopoly rights.
- Moreover, such licences can usually be granted if efforts in obtaining voluntary licences from the patent holders have failed.
- The EU proposal states there that in case of a medical urgency, as is the case now, this condition will be waived.
- The proposal also provides that WTO members would be able to issue compulsory licences even if they do not currently have the provisions to issue them under their national patent laws.
- Compulsory licences can even be granted using executive orders, emergency decrees, and judicial or administrative orders.
Issues with the EU solution
1] Eligible member criteria
- The waiver solution can be used only by an “eligible member”, defined as a “developing country member” of the WTO that “had exported less than 10 percent of world exports of COVID-19 vaccine doses in 2021”.
- This means that Bangladesh, which is still a least developed country, but has a growing pharmaceutical industry, is also excluded.
- Restricting China: The eligibility condition seems to have been introduced to limit China’s expansion in the global vaccine market.
- No concern for India: At the current juncture, India does not have to be concerned with the export restriction clause, as its share in global exports of vaccines was 2.4% as on January 31.
2] Export restrictions in the form of eligibility criteria
- While introducing the above-mentioned export restriction, the solution proposes to waive the obligation under Article 31(f) of the TRIPS Agreement.
- Article 31(f) provides that the compulsory licences issued by any WTO member must be used “predominantly for the supply of the domestic market”.
- But while they have proposed removal of Article 31(f), solution includes a more stringent export restriction in the form of the eligibility criteria mentioned above.
3] Further conditions
- The proposed condition of listing all patents covered under the compulsory licences is not a requirement under the TRIPS Agreement.
- Similarly, there is no obligation to notify the details of licensee, the quantity and export destination under the TRIPS provisions.
- But the EU proposal text proposes mandatory notification.
4] Transfer of know-how is not ensured
- According to the EU, when compulsory licences are granted, the “patent holder receives adequate remuneration”, but “[t]ransfer of know-how is not ensured”.
- This demerit of compulsory licences would make it difficult to scale up production of COVID-19 vaccines, medicines, and medical devices in the developing world, thus constraining their availability at affordable prices.
Conclusion
It must be said that by accepting the “compromise outcome”, India and South Africa could jeopardise their high moral ground. Consequently, the global community would lose an important opportunity to ensure that vaccines and medicines are accessible to all.
UPSC 2022 countdown has begun! Get your personal guidance plan now! (Click here)
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Patent Rights on COVID-19 jabs may be waived
From UPSC perspective, the following things are important :
Prelims level: IPRs, Patents
Mains level: Not Much
The World Trade Organization chief has hailed a breakthrough between the EU, the United States, India and South Africa on waiving intellectual property rights on Covid-19 vaccines.
What is a Patent?
- A patent is an exclusive right granted for an invention.
- In other words, a patent is an exclusive right to a product or a process that generally provides a new way of doing something, or offers a new technical solution to a problem.
- To get a patent, technical information about the invention must be disclosed to the public in a patent application.
- The patent owner may give permission to, or license, other parties to use the invention on mutually agreed terms.
- The owner may also sell the right to the invention to someone else, who will then become the new owner of the patent.
- Once a patent expires, the protection ends, and an invention enters the public domain; that is, anyone can commercially exploit the invention without infringing the patent.
Terms of Patent
- Patents may be granted for inventions in any field of technology, from an everyday kitchen utensil to a nanotechnology chip.
- An invention can be a product – such as a chemical compound, or a process, for example – or a process for producing a specific chemical compound.
- Patent protection is granted for a limited period, generally 20 years from the filing date of the application.
- Patents are territorial rights. In general, the exclusive rights are only applicable in the country or region in which a patent has been filed and granted, in accordance with the law of that country or region.
Back2Basics: Intellectual Properties
- IP is protected in law by, for example, patents, copyright and trademarks, which enable people to earn recognition or financial benefit from what they invent or create.
- By striking the right balance between the interests of innovators and the wider public interest, the IP system aims to foster an environment in which creativity and innovation can flourish.
Types of IP:
(1) Copyright
- Copyright is a legal term used to describe the rights that creators have over their literary and artistic works.
- Works covered by copyright range from books, music, paintings, sculpture and films, to computer programs, databases, advertisements, maps and technical drawings.
(2) Patents
Discussed above
(3) Trademarks
- A trademark is a sign capable of distinguishing the goods or services of one enterprise from those of other enterprises.
- Trademarks date back to ancient times when artisans used to put their signature or “mark” on their products.
(4) Geographical Indications
- Geographical indications and appellations of origin are signs used on goods that have a specific geographical origin and possess qualities, a reputation or characteristics that are essentially attributable to that place of origin.
- Most commonly, a geographical indication includes the name of the place of origin of the goods.
(5) Trade secrets
- Trade secrets are IP rights on confidential information which may be sold or licensed.
- The unauthorized acquisition, use or disclosure of such secret information in a manner contrary to honest commercial practices by others is regarded as an unfair practice and a violation of the trade secret protection.
UPSC 2022 countdown has begun! Get your personal guidance plan now! (Click here)
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Digital Millennium Copyright Act
From UPSC perspective, the following things are important :
Prelims level: Digital Millennium Copyright Act
Mains level: GoI-Twitter row
Union Minister for Electronics and Information Technology was locked out of his Twitter account for an hour allegedly over a notice received for violation of the Digital Millennium Copyright Act (DMCA).
Why such a move by Twitter?
- The DMCA oversees the implementation of two 1996 treaties signed by World Intellectual Property Organization (WIPO) member nations.
What is the DMCA?
- The Digital Millennium Copyright Act, or DMCA, is a 1998 law passed in the US and is among the world’s first laws recognizing intellectual property on the internet.
- The law oversees the implementation of the two treaties signed and agreed upon by member nations of the World Intellectual Property Organization (WIPO) in 1996.
- WIPO members had then agreed upon two treaties, namely the WIPO Copyright Treaty and the WIPO Performances and Phonograms Treaty.
- The said protection, accorded by each member state, must not be any less in any way than the one being given to a domestic copyright holder.
- Further, it also obligates those signatories to the treaty to ensure ways to prevent circumvention of the technical measures used to protect copyrighted work.
- It also provides the necessary international legal protection to digital content.
What is WIPO and how does it ensure the protection of content on the internet?
- The rapid commercialization of the internet in the late 1990s started with static advertisement panels being displayed on the internet.
- It became important for website owners to get the user to spend more time on their webpage.
- For this, fresh content was generated by creators and shared over the Internet.
- The problem started when the content would be copied by unscrupulous websites or users, who did not generate content on their own.
- Further, as the Internet expanded worldwide, websites from countries other than the one where the content originated, also started to copy the unique content generated by the websites.
- To avoid this and bring to task the unauthorized copiers, the members of WIPO, which was established in 1967, also agreed to extend the copyright and intellectual property protection to digital content.
- As of date, 193 nations across the world, including India, are members of WIPO.
Who can generate a DMCA notice and how are they sent to companies or websites?
- Any content creator of any form, who believes that their original content has been copied by the user or a website without authorization can file an application citing their intellectual property has been stolen or violated.
- Users can either approach the website on which the content has been hosted, or third-party service providers like DMCA.com, which utilize a team of experts to help take down the stolen content for a small fee.
- In the case of social media intermediaries like Facebook, Instagram or Twitter, content creators can directly approach the platform with proof of them being original creators.
- Since these companies operate in nations that are signatories to the WIPO treaty, they are obligated to remove the said content if they receive a valid and legal DMCA takedown notice.
- Platforms, however, also give the other users against whom allegations of content cheating have been made, a chance to reply to the DMCA notice by filing a counter-notice.
- The platform shall then decide which party is telling the truth and shall accordingly, either restore the content or keep it hidden.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
How AIDS fight offers a COVID vaccine patent pathway
From UPSC perspective, the following things are important :
Prelims level: TRIPS
Mains level: Paper 3- Ensuring affordability and availability of Covid-19 vaccines
The possibilities of new strain of Covid-19 emerging from any region of the world could derail the global recovery. To prevent that from happening vaccines need to be made available and affordable to all. This article discusses the ways to ensure that.
Ensuring affordability and availability of Covid vaccines
- To achieve global herd immunity and prevent new strains of COVID-19 from emerging, vaccines need to be affordable and available in massive quantities throughout the globe.
- Following three are the ways to ensure vaccine availability and affordability.
- 1) Voluntary linceses: This can happen through patent owners voluntarily licensing their products to other companies, especially Indian producers who are experienced at mass-producing low-cost medications.
- 2) Compulsory licenses: This can also be done by temporarily suspending patent rights for COVID vaccines.
- 3) COVAX option: Some favour ensuring access to COVID-19 vaccines through the COVAX programme.
Options to ensure vaccine availability and affordability
1) Voluntary licencing: Lessons from fight against AIDS
- Due to anti-TRIPS activism from low-income countries and low profits from low-income markets some manufacturers placed licensing agreements to produce AIDS drugs for which they owned patent rights in the UN-affiliated Medicines Patent Pool.
- Several India-based companies then used these voluntary licences to manufacture these drugs on a massive scale and sold them at prices they determined.
- This effort brought down the price of key AIDS medications in these countries.
- The United Nations’ Medicines Patent Pool and the World Health Organization’s COVID-19 Technology Access Pool are important tools in an effort to promote voluntary licensing for COVID products.
- Sharing patent rights through voluntary licensing would need to involve India’s large pharmaceutical sector.
Challenges in voluntary licensing
- So far, no patent holders have joined the WHO’s COVID-19 Technology Access Pool.
- This is why India and South Africa called on the WTO to temporarily waive patent protections for COVID-19.
- Meanwhile, the UN Medicines Patent Pool stands ready to accept voluntary licences for COVID-19.
2) Compulsory licenses
- Compulsory licenses override patent rights to allow local production or import of drugs by generic manufacturers in the event of a public health crisis.
- Since 2003, this right has been enshrined in the Doha Declaration addendum to the WTO’s TRIPS agreement and this is what India and South Africa are lobbying for.
- The Doha addendum, Section 5c, offers AIDS, malaria and tuberculosis as examples of what qualifies as a health emergency.
- By this standard, COVID-19 should easily qualify.
Issues with compulsory licensing
- Good will: Manufacturers in India say they prefer to work with voluntary licences because there is more good will between companies while compulsory licences often come with a legal battle brought by the patent holder.
- Time factor: Voluntary licences also enable production to begin more expeditiously as they usually are accompanied by “technology transfer” meaning that the patent holder reveals to the licensee how to manufacture the medication.
- No need to reverse engineer: Volunatry licensing spares the licensee the lengthy and costly process of figuring out how to reverse engineer the product.
3) COVAX option and issues
- COVAX programme was established to purchase vaccine doses and donate them to low-income countries.
- It does not involve modifying patent rights.
- Underfunded: COVAX is also currently underfunded.
- Delay: The Director-General of WHO warned that people in the lowest-income countries might have to wait until 2022 to get vaccinated through this programme.
Government aid should entail an obligation
- The billions of dollars in government aid given to companies to help develop COVID-19 treatments should entail an obligation to enable the mass production of affordable vaccines.
- Patents are not ironclad ownership rights, they are a temporary contract that balances the public interest with the claims of the innovator.
Consider the question “What is the importance of ensuring availability and affordability of Covid-19 vaccine throughout the world? What are the options available to ensure that?”
Conclusion
This is not just a question of social justice and ensuring life-saving therapies are available to the world’s poor. It is a necessary step to prevent deadlier, more contagious and possibly vaccine-resistant variants of COVID-19 from proliferating in an under-vaccinated world.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
India should walk the talk on TRIPS waiver
From UPSC perspective, the following things are important :
Prelims level: TRIPS
Mains level: Paper 3- TRIPS waiver and India's stand
The article highlights the variance in India’s stand on intellectual property rights waiver for Covid related drugs on the international level and domestic level.
Removing the IPR barrier
- When the pandemic hit the globe, India and South Africa piloted the proposal to waive key provisions of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement on COVID-19 vaccines, drugs, therapeutics, and related technologies.
- The core idea is that IPRs such as patents should not become barriers in scaling up production of medical products essential to combat COVID-19.
- The TRIPS waiver proposal, now backed by the U.S. would give immunity to member countries from a legal challenge at the WTO if their domestic IPR laws suspend or do not enforce IP protection on COVID-19 medical products.
- Member countries of the World Trade Organization (WTO) are under an obligation to ensure that their domestic intellectual property rights (IPR) laws conform to the requirements of the TRIPS agreement.
No use of compulsory licencing in India
- The existing flexibilities under the Patents Act of 1970, such as compulsory licences, which are consistent with the TRIPS agreement, can be used to increase the supply of COVID-19 medical products.
- However, despite the nudging by the judiciary and others, the government inexplicably hasn’t made use of compulsory licences in the pandemic.
- While issuing compulsory licences for COVID-19 vaccines in the absence of technology transfer is easier said than done, they can be used to augment the supply of drugs and other therapeutics.
- For instance, there are demands that compulsory licences be issued for drugs such as Remdesivir to augment supply.
- Natco, an Indian pharmaceutical company, has requested a compulsory licence under Section 92 of the Patents Act for Baricitinib, a COVID-19 drug.
- This is ironic because India has historically played a leading role in mainstreaming TRIPS flexibilities like the compulsory licence at the WTO.
- The Central government, in an affidavit filed before the Supreme Court, states that the main constraint in boosting the production of drugs like Remdesivir is the unavailability of raw materials and essential inputs.
- The affidavit further states, “it is presumptuous to assume that the patent holder will not agree to more voluntary licences”.
Issues with the government’s stand
- If that is the real bottleneck, and not IPR-related legal hurdles, why is India pushing for a TRIPS waiver at the WTO?
- The first step in advocating for the removal of IPR-related impediments at the WTO is to make use of the existing lawful means.
- Therefore, the government’s stand before the Supreme Court is not only contradictory with India’s position at the WTO but also severely undermines it.
Way forward
- To make its TRIPS waiver stand convincing, the government needs to make aggressive use of Sections 92 and 100 of the Patents Act to license all patents necessary to make COVID-19 medical products.
- The government should not only transfer Covaxin’s technology to domestic pharmaceutical companies, to boost national supplies, but also offer it to foreign corporations.
- By unlocking its vaccine technical know-how to the world, India would demonstrate its resolve to walk the talk on the TRIPS waiver.
Conclusion
India must take a consistent stand on IPRs on COVID-19 medical products internationally and domestically.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
A TRIPS waiver is useful but not a magic pill
From UPSC perspective, the following things are important :
Prelims level: Not much
Mains level: Paper 3- Challenges after TRIPS waiver
The article highlights the challenges countries could face despite the patent waiver for Covid-19 vaccine.
TRIPS waiver for Covid-19 vaccine
- The United States has finally relented and declared its support for a temporary waiver of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement for COVID-19 vaccines at the World Trade Organisation (WTO).
- Hopefully, the U.S.’s decision would cause other holdouts like Canada and the European Union to give up their opposition.
- While the U.S.’s decision is to be welcomed, the devil would be in the details.
The challenges after waiver
1) Conditions of the waiver
- If the experience of negotiating such waivers, especially on TRIPS, were anything to go by, it would be too early to celebrate.
- In the aftermath of the HIV/AIDS crisis the WTO adopted a decision in 2003 waiving certain TRIPS obligations to increase the accessibility of medicines.
- However, this waiver (later incorporated as Article 31 bis in the TRIPS agreement) was subject to several stringent requirements such as the drugs so manufactured are to be exported to that nation only; the medicines should be easily identifiable through different colour.
- Given these cumbersome requirements, hardly any country, in the last 17 years, made effective use of this waiver.
2) Countries will protect the interest of pharma companies
- India and South Africa proposed a waiver not just on vaccines but also on medicines and other therapeutics and technologies related to the treatment of COVID-19.
- So, the U.S. has already narrowed down the scope of the waiver considerably by restricting it to vaccines.
- Medicines useful in treating COVID-19 and other therapeutics must be also included in the waiver.
- While the U.S. would not like to be seen as blocking the TRIPS waiver and attracting the ire of the global community, make no mistake that it would resolutely defend the interests of its pharmaceutical corporations.
3) Lack of access to technology
- The TRIPS waiver would lift the legal restrictions on manufacturing COVID-19 vaccines.
- But it would not solve the problem of the lack of access to technological ‘know-how’ related to manufacturing COVID-19 vaccines.
- Waiving IP protection does not impose a legal requirement on pharmaceutical companies to transfer or share technology.
- While individual countries may adopt coercive legal measures for a forced transfer of technology, it would be too draconian and counterproductive.
- Therefore, governments would have to be proactive in negotiating and cajoling pharmaceutical companies to transfer technology using various legal and policy tools including financial incentives.
4) Domestic IP regulation
- While a TRIPS waiver would enable countries to escape WTO obligations, it will not change the nature of domestic IP regulations.
- Therefore, countries should start working towards making suitable changes in their domestic legal framework to operationalise and enforce the TRIPS waiver.
- In this regard, the Indian government should immediately put in place a team of best IP lawyers who could study the various TRIPS waiver scenarios and accordingly recommend the changes to be made in the Indian legal framework.
Conclusion
Notwithstanding the usefulness of the TRIPS waiver, it is not a magic pill. It would work well only if countries simultaneously address the non-IP bottlenecks.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
How IPR served as barrier to the right to access healthcare
From UPSC perspective, the following things are important :
Prelims level: Patent Law, TRIPS
Mains level: Paper 3- Impact of IPR on right to access healthcare
Request for waiver
- Last year, India and South Africa requested WTO for a temporary suspension of rules under the 1995 Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS).
- A waiver was sought to the extent that the protections offered by TRIPS impinged on the containment and treatment of COVID-19.
- The request for a waiver has, since, found support from more than 100 nations.
- But a small group of states — the U.S., the European Union, the U.K. and Canada among them — continues to block the move.
- These countries have already secured the majority of available vaccines.
- But for the rest of the world mass immunisation is a distant dream.
Grounds on which patent laws are justified
- Patent laws are usually justified on three distinct grounds:
- On the idea that people have something of a natural and moral right to claim control over their inventions.
- On the utilitarian premise that exclusive licenses promote invention and therefore benefit society as a whole.
- On the belief that individuals must be allowed to benefit from the fruits of their labour and merit.
- These justifications have long been a matter of contest, especially in the application of claims of monopoly over pharmaceutical drugs and technologies.
Patent laws in India
- In 1959, a committee chaired by Justice N. Rajagopala Ayyangar objected to monopolies on pharmaceutical drugs through colonial-era patent law.
- The committee found that foreign corporations used patents to suppress competition from Indian entities, and thus, medicines were priced at exorbitant rates.
- The committee suggested, and Parliament put this into law through the Patents Act, 1970, that monopolies over pharmaceutical drugs be altogether removed, with protections offered only over claims to processes.
- This change in rule allowed generic manufacturers in India to grow.
How TRIPS goes against the interest of developing countries
- WTO has into its constitution a binding set of rules governing intellectual property.
- Countries that fail to subscribe to the common laws prescribed by the WTO would be barred from entry into the global trading circuit.
- It was believed that a threat of sanctions, to be enforced through a dispute resolution mechanism, would dissuade states from reneging on their promises.
- With the advent in 1995 of the TRIPS agreement, this belief proved true.
- The faults in this new world order became apparent when drugs that reduced AIDS deaths in developed nations were placed out of reach for the rest of the world.
- It was only when Indian companies began to manufacture generic versions of these medicines as TRIPS hadn’t yet kicked in against India, that the prices came down.
Argument in support of the patent regime
- Two common arguments are made in response to objections against the prevailing patent regime.
- One, that unless corporations are rewarded for their inventions, they would be unable to recoup amounts invested by them in research and development.
- Two, without the right to monopolise production there will be no incentive to innovate.
Issues with the argument in support of patent regime
- Big pharma has never been forthright about the quantum of monies funnelled by it into research and development.
- Moderna vaccine in the U.S. emanated out of basic research conducted by the National Institutes of Health, a federal government agency, and other publicly funded universities and organisations.
- Similarly, public money accounted for more than 97% of the funding towards the development of the Oxford/AstraZeneca vaccine.
- Therefore, the claim that the removal of patents would somehow invade on a company’s ability to recoup costs is simply untrue.
- The second objection — the idea that patents are the only means available to promote innovation — has become something of a dogma.
- The economist Joseph Stiglitz is one of many who has proposed a prize fund for medical research in place of patents.
Consider the question “What are the issues with the patent regime under the TRIPS in the field of medicine?”
Conclusion
We cannot continue to persist with rules granting monopolies which place the right to access basic healthcare in a position of constant peril. In its present form, the TRIPS regime represents nothing but a new form of “feudal calculus”.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
[pib] KAPILA for patent awareness
From UPSC perspective, the following things are important :
Prelims level: KAPILA program
Mains level: Patenting solutions in India
The Government has launched a campaign namely KAPILA for Intellectual Property Literacy and creating patent awareness, informed Union Ministry for Education to the Parliament.
Remember one thing, ‘KAPILA’ Program is related to IP awareness. It sounds much like an animal husbandry related initiative.
KAPILA Initiative
- KAPILA is an acronym for Kalam Program for IP (Intellectual Property) Literacy and Awareness.
- Under this campaign, students pursuing education in higher educational institutions will get information about the correct system of the application process for patenting their invention and they will be aware of their rights.
- The program will facilitate the colleges and institutions to encourage more and more students to file patents.
Why in news?
- As many as 46,556 users have registered for the Union Government’s Intellectual Property Literary project.
- This marks the success of the campaign.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
PATENTS (AMENDMENT) RULES, 2020
From UPSC perspective, the following things are important :
Prelims level: Patent Act 1970
Mains level: Paper 3- Amendment to patent working disclosure rule and its implications
A recent amendment to a unique feature in patent law under which patentee/licensee has to disclose information regarding the extent to which they have worked patent in India, could have several implications.
Why the changes in rules matter
- Indian patent law grants a 20-year patent monopoly to an inventor.
- In exchange for such monopoly, India’s patent law imposes a duty on the patentee to commercially work the invention in India to ensure that its benefits reach the public.
- Accordingly, section 146(2), a unique provision not found in patent laws of most other countries, requires every patentee and licensee to submit to the Patent Office an annual statement (Form 27 format) explaining the extent to which they have worked the invention in India.
- This statement is meant to help the Patent Office, potential competitors, etc. to determine whether the patentee has worked the invention in India and made it sufficiently available to the public at reasonable prices.
- A failure of this duty could trigger compulsory licensing or even subsequent revocation of the patent under the Patents Act, 1970.
- The central government recently amended the format of a statement that patentees and licensees are required to annually submit to the Patent Office.
- The amendment has significantly watered down the disclosure format.
- This could hamper the effectiveness of India’s compulsory licensing regime.
- This in turn could hinder access to vital inventions including life-saving medicines, thereby impacting public health.
- There has been significant pressure from multinational corporations and the United States government to do away with this requirement.
What changes were made through the amendment
- The recent amendment to the form was made in response to a PIL filed by Shamnad Basheer before the Delhi High Court in 2015.
- The PIL brought to the Court’s attention the rampant non-filing and defective filing of Form 27 and sought a direction to strictly enforce the patent working disclosure rules and take action against the violators.
- The PIL also called for a reform of Form 27, arguing that the information it sought was grossly insufficient to ascertain the extent of the working of the patent.
- However, instead of strengthening the form, the amendment has significantly weakened it further, thereby defeating the entire purpose of the amendment exercise.
- The amended form has removed the requirement of submitting a lot of important information.
- It is no longer required to provide any information in respect of the quantum of the invention manufactured/imported into India, the licenses and sub-licenses granted during the year and the meeting of public requirement at a reasonable price.
- It no longer requires quantum or the total units of the invention manufactured/imported in India.
- The deletion of this requirement of its disclosure is shocking.
- This is because, it is the disclosure of this data by Bayer in Form 27 that played a crucial role in grant of India’s first compulsory license to Natco for the anti-cancer drug Sorafenib/Nexavar.
- The removal of the requirement of submitting any licensing information, including the disclosure of even the existence of licenses means that the patentees/licensees can just self-certify that they’ve worked the patent.
- The omission to mandate disclosure of details makes it extremely difficult to ascertain whether the invention has been made available to the public in sufficient quantity and at an affordable price.
Conclusion
The government has significantly weakened the critical duty imposed by the law on patentees/licensees to disclose patent working information. Therefore, the government must reconsider its amendments to the form taking into account the PIL recommendations and re-amend it to restore as well as strengthen its spirit.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
[pib] KAPILA Program
From UPSC perspective, the following things are important :
Prelims level: KAPILA program
Mains level: IPR protection measures
Union Education Ministry has launched ‘KAPILA’ Kalam Program for IP Literacy and Awareness Education campaign to bring awareness towards the patenting of inventions.
Remember one thing, ‘KAPILA’ Program is related to IP awareness. It sounds much like an animal husbandry related initiative.
‘KAPILA’ Program
- KAPILA is an acronym for Kalam Program for IP (Intellectual Property) Literacy and Awareness.
- Under this campaign, students pursuing education in higher educational institutions will get information about the correct system of the application process for patenting their invention and they will be aware of their rights.
- The program will facilitate the colleges and institutions to encourage more and more students to file patents.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Rethinking the role of Intellectual Property in Corona crisis
From UPSC perspective, the following things are important :
Prelims level: TRIPS.
Mains level: Paper 3- How Covid-19 could impact the intellectual property rights?
The article discusses the idea of creating a patent pool of the patents dealing with Covid-19. Such a patent pool will be effective in avoiding the possibility of the hostile response of societies towards patent rights. And also avoid the conflict between nations. corporations and international organisations.
Purpose of patent rights
- The purpose of creating and recognising patent rights is for the common public good, i.e., innovation should be made public in exchange for a limited monopoly.
- Thus, patents need to be disclosed to the public in order to enable further research.
- Should pandemics such as COVID-19be an exception to this?
- With the outbreak of COVID-19, there are several innovations.
- All these innovations may be the subject matter of patent applications around the world.
- It will be a few years before patents are even granted.
- However, friction already exists among various stakeholders.
- For instance, one country made attempts to obtain exclusive rights to a vaccine being developed.
- On the other hand, there are also collaborations taking place.
- However, the spirit of collaborative solutions is only on the anvil.
- The question that arises is whether the exclusivity that is recognised by patent rights will be detrimental to society.
- Will patents create roadblocks or is there a solution?
Possibility of conflicts over patent rights
- Governments and international organisations need to arrive at a consensus in advance to ensure that the system is ready.
- Procrastination would be disastrous.
- Creating hindrances through exclusivity claims, in the wake of a pandemic, will result in dividing countries, corporations and international organisations.
- This will not benefit patients and the world as a whole.
- If patent owners create impediments on the strength of patent rights, the world will start despising patents and that is not a situation IP owners ought to be in.
- Under the TRIPS (Trade-Related Aspects of Intellectual Property Rights) regime, there are several tools such as compulsory licensing that are available to ensure access to medicines.
- However, beyond the laws, society needs to respect innovation.
- To protect the sanctity and integrity of patent systems, and in order to ensure that an anti-IP sentiment is not generated globally, answers need to be found within the existing regime.
- In exceptional circumstances such as these, there is a likelihood that societies may resort to extreme steps to protect themselves.
- Before such ideas are floated, solutions should be created.
The idea of creating a patent pool
- One method by which aggregation and dissemination of innovative products can be ensured is by creating a patent pool.
- Patent pools are usually effective in aggregating, administering and licensing patents related to specific areas of technology.
- Such pools are usually managed by a central agency and the patents which become part of the pool are readily made available for licensing.
- Some pools even publish the royalty rates payable for such licences.
- Anyone who wishes to obtain a licence will be able to approach the pool, agree to the terms, and begin to manufacture and sell the products.
- Such pools are prevalent in, for instance, standard essential patents related to telecom and digital innovations.
- At the moment, individual efforts are being made by research organisations to create their own pools.
- A more fruitful endeavour would be to create a global pool of COVID-19-related innovations, or innovations related to rare pandemics, in respect of vaccines and medicines.
- This could be managed by a trustworthy international organisation.
- All countries ought to have the right to implement these innovations without further permission from the patent-holders.
- This would not require countries resorting to provisions such as compulsory licensing, state acquisition, etc.
- Even if royalties are at a minimal level, the revenues would still be in billions of dollars owing to the large swathes of the population affected by the pandemic, who will need to be administered these products.
Way forward
- Creation of a pool and immediate licensing will ensure that there are hundreds of manufacturers across the world.
- As a result, vaccines and medicines will be quickly available.
- Such a pool needs the cooperation of not just countries and international organisations but also the hundreds of researchers, innovators, companies and universities involved.
- Doha Declaration: Pooling of patent resources is also in line with the Doha Declaration on Public Health which is a part of the TRIPS agreement.
- This declaration recognises the need for taking measures to ‘protect public health’ and ‘promote access to medicines’.
A direct question on the issue can be asked by the UPSC, for ex-“Though IPRs have been provided to respect and protect the innovations and ideas, but in the wake of corona crisis, some strict provisions need to be changed. In light of the above statement, discuss the limitations of the exclusivity clause under the patents rights. And how can it be overcome in emergency situations?”
Conclusion
Public-private partnerships (PPP) need to be scaled up. Creation of the ‘PPP-pandemic patent pool’ at a global level, to pool all innovations, is the way forward. Let us not wait any longer.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
Intellectual Property Rights in India
Global Intellectual Property Index 2020
From UPSC perspective, the following things are important :
Prelims level: Prospects of the Global IP Index
Mains level: Intellectual property rights and their protection in India
India has been ranked 40th out of 53 countries on a global intellectual property index, even as the country has shown improvement in terms of scores when it comes to the protection of IP and copyright issues.
GIP Index
- The Global IP Index was released by Global Innovation Policy Center or GIPC of the US Chambers of Commerce.
- The GIPC Index consists of five key sets of indicators to map the national intellectual property environment for the surveyed countries.
- The major indicator categories are:
- patents, related rights, and limitations;
- copyrights, related rights, and limitations;
- trademarks, related rights, and limitations;
- enforcement;
- membership and ratification of international treaties.
India’s performance
- India was placed at 36th position among 50 countries in 2019.
- India’s score, however, increased from 36.04 per cent (16.22 out of 45) in 2019 to 38.46 per cent (19.23 out of 50) in 2020, a 2.42 per cent jump in absolute score.
- However, India’s relative score increased by 6.71 per cent.
- India also continues to score well in the Systemic Efficiency indicator, scoring ahead of 28 other economies in these indicators.
Challenges for India
- GIPC has identified several challenges for India. Prominent among them are:
Patentability requirements, patent enforcement, compulsory licensing, patent opposition, regulatory data protection, transparency in reporting seizures by customs, and Singapore Treaty of Law of TMs and Patent Law Treaty
Measures to protect IPs in India
- Since the release of the 2016 National IPR Policy, the government of India has made a focused effort to support investments in innovation and creativity through increasingly robust IP protection and enforcement.
- Since 2016, India has improved the speed of processing for patent and trademark applications, increased awareness of IP rights among Indian innovators and creators, and facilitated the registration and enforcement of those rights.
- To continue this upward trajectory, much work remains to be done to introduce transformative changes to India’s overall IP framework and take serious steps to consistently implement strong IP standards.
Get an IAS/IPS ranker as your 1: 1 personal mentor for UPSC 2024
What are IPRs?
Intellectual Property Rights (IPRs) are legal rights, which result from intellectual invention, innovation and discovery in the industrial, scientific, literary and artistic fields. These rights entitle an individual or group to the moral and economic rights of creators in their creation.
Types:
Patent- It is a set of exclusive rights granted by a sovereign state to an inventor for a limited period of time in exchange for detailed public disclosure of an invention.
Copyright- It is a legal right created by the law of a country that grants the creator of an original work exclusive rights for its use and distribution. It includes literary & artistic works such as novels, poems, plays, films, musical works, drawing, painting, photography, sculpture, architectural designs
Trademark- It is a recognizable sign, design, or expression which identifies products or services of a particular source from those of others. Trademarks used to identify services are usually called service marks.
Industrial design right- It is an intellectual property right that protects the visual design of objects that are not purely utilitarian. An industrial design consists of the creation of a shape, configuration or composition of pattern or color, or combination of pattern and color in three-dimensional form containing aesthetic value. An industrial design can be a two- or three-dimensional pattern used to produce a product, industrial commodity or handicraft.
Trade secret- It is a formula, practice, process, design, instrument, pattern, commercial method, or compilation of information which is not generally known or reasonably ascertainable by others, and by which a business can obtain an economic advantage over competitors or customers
Geographical Indication (GI)- It is a name or sign used on certain products which corresponds to a specific geographical location or origin (e.g. a town, region, or country). The use of a geographical indication may act as a certification that the product possesses certain qualities, is made according to traditional methods, or enjoys a certain reputation, due to its geographical origin. A recent example is of Indian variety of Basmati rice getting GI tag.
From above points, it is clear that IPR is a very sensitive issue in terms of businesses different kinds and international relations as well.
IPRs in pharmaceutical sector:
Some sectors are very sensitive in terms of IPRs like pharmaceuticals. Let’s explore briefly into IPR issues in pharmaceutical sector.
We hear of two kinds of drugs- generic and brand name drugs:
Generic drugs are those whose patent has expired or does not exist and which can be produced by any registered manufacturer without need of taking permission from any authority and also without any payment of royalty.
Brand name drugs are those which are patented and cannot be produced without the consent of the patent holder. A royalty is to be paid for production of these drugs.
But what happens if a company holds patent of an essential drug and there is an emergency in which the drug needs to be provided at low cost for vast populace? In this case, Compulsory Licensing comes to the rescue.
What is Compulsory Licensing?
- A compulsory license provides that the owner of a patent or copyright licenses the use of their rights against a payment. This payment is either set by law or determined through some form of arbitration
- In essence, under a compulsory license, an individual or company seeking to use another’s intellectual property can do so without seeking the rights holder’s consent, and pays the rights holder a set fee for the license
- This is an exception to the general rule under intellectual property laws that the intellectual property owner enjoys exclusive rights that it may license – or decline to license – to others
Does there have to be an emergency?
Not necessarily. This is a common misunderstanding. The TRIPS Agreement does not specifically list the reasons that might be used to justify compulsory licensing. However, the Doha Declaration on TRIPS and Public Health confirms that countries are free to determine the grounds for granting compulsory licences.
In March 2012, India granted its first compulsory license ever. The license was granted to Indian generic drug manufacturer Natco Pharma Ltd for Sorafenib tosylate, a cancer drug patented by Bayer.
Here, first thing first, What is TRIPS?
- TRIPS is an international agreement administered by the World Trade Organization (WTO), which sets down minimum standards for many forms of intellectual property (IP) regulations as applied to the nationals of other WTO Members
- It was negotiated at the end of the Uruguay Round of the General Agreement on Tariffs and Trade (GATT) in 1994
- TRIPS requires WTO members to provide copyright rights, covering content producers including performers, producers of sound recordings and broadcasting organizations, geographical indications, including appellations of origin, industrial designs, integrated circuit layout-designs, patents, new plant varieties, trademarks, trade dress, and undisclosed or confidential information
- The agreement also specifies enforcement procedures, remedies, and dispute resolution procedures
Now, back to the topic…
India is a huge market for generic drugs and hence it is very obvious that there must emerge issues out of patents for pharmaceuticals.
One such case came up in 1998- Novartis v. Union of India & Others
It was a landmark decision by a two-judge bench of the Supreme Court, on the issue of whether Novartis could patent Glivec in India. It was the culmination of a seven-year-long litigation fought by Novartis. The Supreme Court upheld the Indian patent office’s rejection of the patent application.
Ground of rejection?
Novartis claimed patent for he changed form of Glivec on the basis of the increased bio-availability in the body of the patient by making changes in chemical composition of its original anti-cancer drug Imatinib Mesylate. This changed form of the drug could not withstand the ‘enhanced therapeutic efficacy’ test enshrined under Section 3(d) of Indian Patents Act and therefore it was rejected.
Recently, Gilead got patent for its Hepatitis C drug Solvadi. An application for the same patent was first rejected in January 2015 as lacking inventiveness and novelty. The decision, however, is seen as a major blow to the access to drug movement
Now let’s turn towards the latest developments in the IPRs in India.
New IPR Policy
Govt of India recently released a new National Intellectual Property Rights (IPR) Policy which is in compliance with WTO’s agreement on TRIPS
Why a new policy?
- Global drug brands led by US companies have been pushing for changes to India’s intellectual property rules for quite some time now. They have often complained about India’s price controls and marketing restrictions
- Also, an IPR policy is important for the government to formulate incentives in the form of tax concessions to encourage research and development (R&D)
- It is also critical to strengthen the Make In India, Startup and Digital India schemes
- The IPR policy comes at a time when India and other emerging countries faces fresh challenges from the developed world and mega regional trade agreements such as the Trans-Pacific Partnership (TPP)
Seven objectives:
- IPR Awareness: To create public awareness about the economic, social and cultural benefits of IPRs among all sections of society
- Generation of IPRs: To stimulate the generation of IPRs
- Legal and Legislative Framework: To have strong and effective IPR laws, which balance the interests of rights owners with larger public interest
- Administration and Management: To modernize and strengthen service-oriented IPR administration
- Commercialization of IPRs: Get value for IPRs through commercialization
- Enforcement and Adjudication: To strengthen the enforcement and adjudicatory mechanisms for combating IPR infringements
- Human Capital Development: To strengthen and expand human resources, institutions and capacities for teaching, training, research and skill building in IPRs
Highlights:
- The new policy calls for providing financial support to the less empowered groups of IP owners or creators such as farmers, weavers and artisans through financial institutions like rural banks or co-operative banks offering IP-friendly loans
- The work done by various ministries and departments will be monitored by the Department of Industrial Policy & Promotion (DIPP), which will be the nodal department to coordinate, guide and oversee implementation and future development of IPRs in India
- The policy, with a tagline of Creative India: Innovative India, also calls for updating various intellectual property laws, including the Indian Cinematography Act, to remove anomalies and inconsistencies in consultation with stakeholders
- For supporting financial aspects of IPR commercialisation, it asks for financial support to develop IP assets through links with financial institutions, including banks, VC funds, angel funds and crowd-funding mechanisms
- To achieve the objective of strengthening enforcement and adjudicatory mechanisms to combat IPR infringements, it called for taking actions against attempts to treat generic drugs as spurious or counterfeit and undertake stringent measures to curb manufacture and sale of misbranded, adulterated and spurious drugs
- The policy will be reviewed after every five years to keep pace with further developments in the sector
International angle:
Last month, the US Trade Representative kept India, China and Russia on its “Priority Watch List” for inadequate improvement in IPR protection. However, brushing aside concerns of the US on India’s IPR regime, the government said its intellectual property rights laws are legal-equitable and WTO-compliant. Thus, the government has not yielded to pressure from the United States to amend India’s patent laws.
Benefits:
- The new policy will try to safeguard the interests of rights owners with the wider public interest, while combating infringements of intellectual property rights
- By 2017, the window for trademark registration will be brought down to one month. This will help in clearing over 237,000 pending applications in India’s four patent offices
- It also seeks to promote R&D through tax benefits available under various laws and simplification of procedures for availing of direct and indirect tax benefits
- Unlike earlier where copyright was accorded to only books and publications, the recast regime will cover films, music and industrial drawings
- A host of laws will also be streamlined — on semi-conductors, designs, geographical indications, trademarks and patents
- The policy also puts a premium on enhancing access to healthcare, food security and environmental protection
- Policy will provide both domestic and foreign investors a stable IPR framework in the country
- This will promote a holistic and conducive ecosystem to catalyse the full potential of intellectual property for India’s growth and socio-cultural development while protecting public interest
- It is expected to lay the future roadmap for intellectual property in India, besides putting in place an institutional mechanism for implementation, monitoring and review
- The idea is to incorporate global best practices in the Indian context and adapt to the same
Challenges:
- According to the policy, India will retain the right to issue so-called compulsory licenses to its drug firms, under “emergency” conditions
- Also, the government has indicated that there is no urgent need to change patent laws that are already fully World Trade Organization-compliant. So India has resisted pressure from the US and other Western countries to amend its patent laws
- The policy also specifically does not open up Section 3(d) of the Patents Act, which sets the standard for what is considered an invention in India, for reinterpretation
Published with inputs from Swapnil